第一作者简介: 尚墨翰,男,1993年生,古生物学与地层学博士研究生。E-mail: shangmohan126@126.com。
海水氧化还原条件显著影响真核生物的起源与早期演化,但以往有关早期海水氧化还原条件研究的对象,主要依赖相对深水的细粒碎屑岩沉积(如黑色页岩),而对真核生物集中分布的浅水环境中的碳酸盐岩关注不够且手段缺乏。这显著制约了对真核生物起源与早期演化机理的认识。近年来,有学者提出碳酸盐岩的 I/( Ca+ Mg)值可作为反映海洋氧化还原条件的重要指标,并将其广泛应用于海相碳酸盐岩的古氧相研究中。该指标的提出主要基于对现代海洋碘组分的观测以及室内方解石合成实验结果: 观测结果表明,海水中的碘主要以氧化态( IO3-)和还原态( I-) 2种形式存在,随着氧含量的下降(如在氧极小带),氧化态的碘被逐步转换为还原态的碘,且海水中的 IO3-浓度与海水氧含量大体呈正相关。实验研究证明, IO3-可按一定的分配系数进入到碳酸盐矿物晶格中,但 I-则不能。由于 IO3- /I-的还原势能与 O2/H2O的还原势能接近,因此 I/( Ca+ Mg)值是最早响应海洋氧含量下降的指标之一,可用于表征深时(如前寒武纪)次氧化环境中表层海水的氧含量波动。此外,学者们也尝试建立 I/( Ca+ Mg)值与氧含量之间的半定量关系,如 I/( Ca+ Mg)值大于 0和 2.5μmol/mol这两个临界值所对应的海水氧含量。结合大量现代缺氧水体和氧极小带中碘组分与溶解氧浓度相关关系的研究,作者提出 I/( Ca+ Mg)= 1.5μmol/mol为重要的临界值之一,可用于限定初级生产力在表层海水中所能产生的最大氧浓度值( ~10 μM),并能进一步区分海水和大气的氧化。此外,对 I/( Ca+ Mg)值的应用进展及潜在问题进行评述,并对可能的发展方向进行展望。
About the first author: Shang Mo-Han,born in 1993,is a Ph.D. candidate of paleontology and stratigraphy. E-mail: shangmohan126@126.com.
Fund:The redox conditions of seawater play a pivotal role in influencing the origin and early evolution of eukaryotes. However,previous studies regarding ocean redox conditions mainly focus on fine-grained siliciclastic rocks(e.g.,black shale)deposited in relatively deep seawater,rather than carbonates formed in eukaryote-concentrated shallow seawater,due largely to a lack of valid method,significantly limiting our understanding of the mechanisms concerning the origin and early evolution of eukaryotes. In recent years,I/(Ca+Mg)was proposed as a proxy for redox conditions of seawater,and has been widely employed in carbonates to analysis seawater redox conditions. The proposal of this proxy is mainly based on measurements of iodine speciation in modern oceans and experiments of calcite synthesis in laboratory. The measurements demonstrate that marine iodine composition mainly occur in two states, namely, Oxidized-state iodate(IO3-)and reduced-state iodide(I-). With the decrease of oxygen concentration(such as in an oxygen minimum zone,OMZ),the oxidized-state iodate,which is proportional to the oxygen concentration,would be gradually reduced into reduced-state iodide. The experiments confirm that only IO3- could be incorporated into the lattices of carbonate minerals with a fixed distribution coefficient,but I-would be excluded. Because of the high redox potential of IO3-/I-,which is close to that of O2/H2O,I/(Ca+Mg) is one of the proxies earliest responding to the decrease of ocean oxygen concentration. I/(Ca+Mg) is therefore sensitive to the variation of oxygen concentrations in weakly oxidized surface seawaters in deep time(e.g.,Precambrian). Furthermore,some scholars attempted to establish semiquantitative relationships of I/(Ca+Mg)values to oxygen concentrations,and two threshold values of I/(Ca+Mg)>0 and 2.5μmol/mol have been proposed as the semiquantitative constraints for the oxygen concentrations in ancient ocean waters. In addition,in the light of the study of iodine speciation and dissolved oxygen concentrations in modern anoxic basins and water columns within OMZs,our results suggest that I/(Ca+Mg)=1.5μmol/mol could be used as the threshold between atmosphere and surface seawater. This threshold value may be used to reflect that the oxygen concentration of surface ocean is up to 10 μM,which is the maximum oxygen concentration increased by the primary productivity,and therefore to distinguish the potential variations of oxygen concentration between atmosphere and surface seawaters. In this paper,some of the recent progress and potential problems in redox analysis using I/(Ca+Mg)in ancient carbonates were briefly reviewed,and some tentative suggestions for future study were also put forward.
海水的化学条件对真核生物的起源和演化具有重要影响(例如, Knoll and Carroll, 1999; Anbar and Knoll, 2002; Glass et al., 2009; Guan et al., 2014), 尤其是海水的氧化还原条件, 直接控制了新元古代晚期后生动物的兴起与生态分布(例如, Knoll and Carroll, 1999; Canfield et al., 2007; Sahoo et al., 2012, 2016)。因此, 针对元古代海水氧含量进行研究, 将对认识真核生物和后生动物的起源和早期演化具有重要的科学意义。尽管近年来对早期大气(Partin et al., 2013; Planavsky et al., 2014)和海洋(Johnston et al., 2010; Li et al. 2010; Poulton et al., 2010; Planavsky et al., 2011; Poulton and Canfield, 2011; Canfield et al., 2013; Partin et al., 2013; Reinhard et al., 2013)氧含量的研究取得了重大进展和新认识, 但对许多时段的认识目前仍限于理论推测(Planavsky et al., 2011; Reinhard et al., 2013)且争议很大(Planavsky et al., 2014; Cole et al., 2016; Gilleaudeau et al., 2016; Tang et al., 2016; Zhang et al., 2016, 2017; Hardisty et al., 2017)。而在分析方法上, 对海水氧化还原的研究主要运用的是铁组分(例如, Poulton et al., 2004, 2010; Canfield et al., 2007, 2008; Li et al., 2010; Planavsky et al., 2011)、氧化还原敏感元素丰度(例如, Scott et al., 2008; Scott and Lyons, 2012; Reinhard et al., 2013; Wang et al., 2015b)、N同位素(例如, Stü eken, 2013, 2016; Ader et al., 2014; Wang et al., 2015a; Koehler et al., 2017)、Cr同位素(例如, Frei et al., 2009; Planavsky et al., 2014)和Mo同位素(例如, Arnold et al., 2004; Kendall et al., 2015; Kurzweil et al., 2015)等。这些方法主要适用于相对深水的细粒碎屑岩样品(如页岩), 而对于广泛发育的浅水碳酸盐岩, 仍缺乏有效的反映周围海水氧化还原状态的地球化学指标。事实上, 早期真核生物的生态空间可能被局限在氧含量相对较高的浅水区域(Adam, 2014)。因此, 针对浅水碳酸盐岩所记录的水体氧化还原状态的研究, 将为探讨海水氧化还原状态以及真核生物与后生动物的演化提供重要、直观的信息。
早期针对浅水碳酸盐岩的氧化还原研究, 采用的指标主要为稀土元素Ce异常, 但是该指标极易受到陆源碎屑尤其是黏土矿物的影响。已有的一些研究结果表明, 那些陆源碎屑含量较高的碳酸盐岩样品, 主要记录的是碎屑的稀土组成, 而非海水的氧化还原信号(例如, Planavsky et al., 2010; Ling et al., 2013; Tang et al., 2016)。对现代海洋中氧极小带和缺氧盆地碘(I)组分的早期观测表明, 随着O2的耗尽, 碘酸根离子(I
为更好地应用这一指标, 文中总结了I/(Ca+Mg)值作为氧化还原指标的基本原理、碳酸盐岩中碘含量的测试方法、I/(Ca+Mg)值中若干重要临界值的古环境氧含量指示意义、前寒武纪海洋中I/(Ca+Mg)值的长期演变特征以及将这一指标作为古代碳酸盐岩氧化还原指标可能存在的问题, 并对下一步的研究方向予以展望。
现代海洋的观测表明, 虽然碘元素能以多种价态存在, 但具有热稳定性的溶解无机碘仅有氧化态的碘酸根离子(I
碘酸盐是一种海洋生物的微营养物质(Kü pper et al., 2010), 初级生产力和有机质降解可分别导致表层海水中少量至~50%碘酸盐的损失以及深层水柱中碘化物的析出。这种情况在现代海洋的许多区域均可观察到, 包括夏威夷和百慕大群岛(Campos et al., 1996)、威德尔海(Campos et al., 1999; Bluhm et al., 2011)、地中海(Tian et al., 1996)、阿拉伯海(Farrenkopf and Luther Ⅲ , 2002)及南极洲沿岸水域(Chance et al., 2010)等。另一方面, 深部氧化水体的上涌可补偿表层海水中碘酸盐的减少(Truesdale and Bailey, 2002)。因此, 尽管受生物摄取/释放的影响, 但仍普遍认为海水中碘组分的主要控制因素为局部海水的氧化还原状态。目前已知I
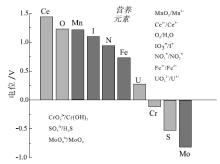 | 图 1 各种元素的还原电位和氧化还原对(据Lu et al., 2010, 有修改)Fig.1 Reduction potentials and redox couples of various elements(modified from Lu et al., 2010) |
每一种氧化还原指标, 均在不同的电位范围内响应环境的变化。那些具有低还原电位的指标(如, Mo
碳酸盐岩的碘含量测试分析, 在国内开展的相对较少, 但在国际上已有较为成熟的方法。现有的标准方法中, I/(Ca+Mg)值可使用四级杆电感耦合等离子体质谱仪测量(ICP-MS, 如Bruker M90)(Lu et al., 2010, 2016; Hardisty et al., 2014, 2017; Zhou et al., 2014, 2015):首先, 称3~5mg样品粉末, 用1mL去离子水漂洗4次, 并离心倾析上层清液, 以去除样品表面可能附着的可溶性碘盐。然后, 配加3%硝酸并置于超声水浴中~10min, 以完全溶解碳酸盐; 残留的非碳酸盐杂质通过离心去除, 保留上层清液。最后, 溶液被稀释至含有~50 μ g/g的Ca、~0.5%的三甲胺(用于稳定碘酸盐)(Schnetger and Muramatsu, 1996)以及内标(如In)。在ICP-MS测试过程中, 对于每个批次的测试, 均采用不同浓度的分析纯碘酸钾建立标准曲线。
当使用Bruker M90型ICP-MS上机测试时, 应首先将设备的灵敏度调至1 ng/g浓度的127I所对应的设备计数率为~80-100 kcps。测试结果表明, 127I的精确度普遍优于1%, 长期准确性可通过参考物质监控, 如Jcp-1(Lu et al., 2010)。该标样国内较难获得, 可使用GSR-12(GBW07114)替代。I/(Ca+Mg)值的检测限通常低于0.1μ mol/mol。对于灵敏度较低的ICP-MS, 可以提高样品质量至40mg, 减少溶液稀释倍率至含有~400 μ g/g的Ca, 灵敏度调至1 ng/g浓度的127I所对应的设备计数率为~2-3 kcps, 此时测试的127I精确度可普遍优于6%。需要注意的是, 本处理方法仅限于受黏土、硅酸盐及有机质影响较小的碳酸盐岩, 因为在化学分析时, 附着于非碳酸盐岩的碘有可能影响所测试的I/(Ca+Mg)值。若难以筛选出符合条件的样品, 需要评估非碳酸盐岩对I/(Ca+Mg)值的影响。
研究表明, 未遭受显著成岩作用改造的碳酸盐岩, 若其具有高的I/(Ca+Mg)值, 便可明确指示沉积水体具有高氧气含量(例如, Lu et al., 2016)。尽管全球标定结果表明I/(Ca+Mg)值并不能完全线性、定量地反映水体溶解氧浓度, 但基于现代海洋碘组分的研究成果, 笔者依然能够确定一组I/(Ca+Mg)的临界值用于半定量地限定沉积水体的溶氧量(例如, Hardisty et al., 2014, 2017; Lu et al., 2016)。
前人指出, 海洋中I
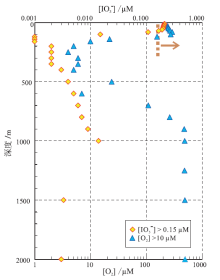 | 图 2 秘鲁远海OMZ中溶解的[I |
依据这一指标, Hardisty等(2014)提出, GOE期间海水表面氧气浓度高于1 μ M。另外, Hardisty 等(2017)指出, 大部分元古宙碳酸盐岩单元的I/(Ca+Mg)> 0μ mol/mol, 是表层海水[O2]> 1~3μ M的有力证据。这一认识得到了中元古界发现真核生物微体化石的支持(Knoll, 2014), 也得到了模拟结果的支持, 即在完全缺氧的大气环境下, 表层水体内的光合作用也可导致氧气浓度升高至数微摩尔浓度(Reinhard et al., 2016)。
区分海水氧化与大气氧化十分必要, 因为二者的氧化一般并不完全同步。研究表明, 当大气氧含量小于 2.5% PAL时, 表层海水的氧含量主要受控于初级生产力, 从而很难与大气达到平衡(Reinhard et al., 2016)。换言之, 当大气氧含量小于2.5% PAL时, 表层海水的氧化还原状态并不能直接指示大气氧的含量。但是, 模型研究表明, 初级生产力所导致的局部氧气浓度升高仅限于1~10 μ M之间, 不可能超过10 μ M(Kasting, 1991; Olson et al., 2013; Reinhard et al., 2013)。因此, 建立表层海水氧浓度为10μ M时对应的海水I
由于受生物吸附作用及其之下水体是否存在氧极小带等因素的影响, 表层海水的I
I
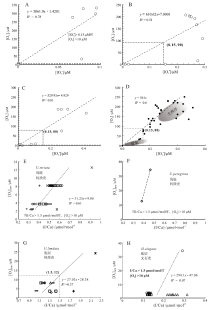 | 图 3 低氧水体中[I A— 黑海[I |
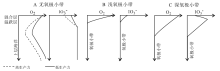 | 图 4 海水碘酸盐和氧浓度变化示意图(据Zhou et al., 2014修改)Fig.4 Schematic seawater iodate and oxygen concentration profiles(modified from Zhou et al., 2014) |
就存在OMZ条件下的I
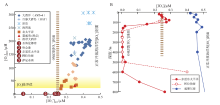 | 图 5 现代开放海洋和具OMZ海洋的表层水体I A— 现代海洋表层海水中I |
碳酸盐岩中I/(Ca+Mg)值的长期演化序列表明, 元古宙碳酸盐岩中I/(Ca+Mg)平均值较显生宙偏低(图 6)。Hardisty等(2017)认为这一特征指示, 在元古宙大部分时期表层海水仅微弱氧化, 且在邻近区域存在缺氧水体。这可能说明, 元古宙海洋表层水体的氧化还原状态, 类似于现代海洋的缺氧盆地和OMZ附近的氧跃层(图 2)。在这些环境中游离氧可局部存在, 但同时在该部位或相邻地区的可交换水体中, I
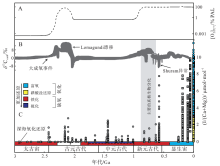 | 图 6 海洋I/(Ca+Mg)值的长期演变趋势 A— 大气氧含量长期演化趋势(Lyons et al., 2014; Planavsky et al., 2014), 虚线表示缺乏定量约束的不确定性; B— |
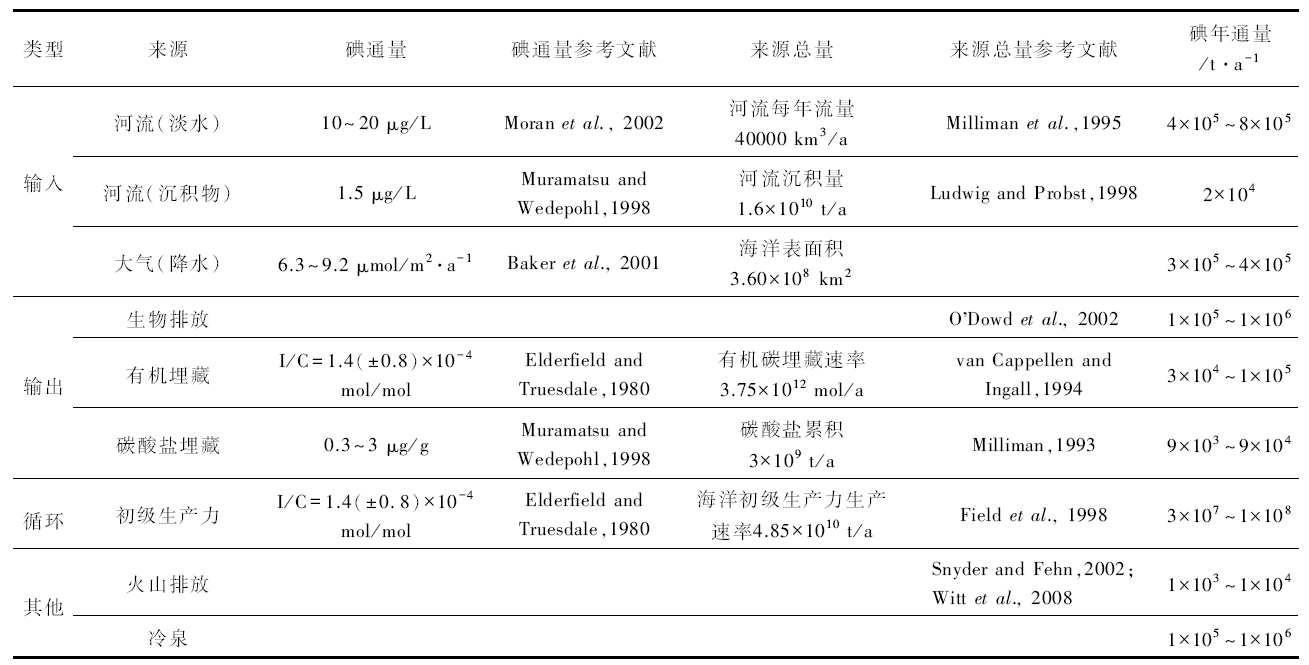
虽然现代海洋碘库的大小尚未被精确限定(约为0.45 μ M), 但据估算, 进出海水的碘通量(如河流及地壳的输入、有机质埋藏的输出), 可能比生物生产力相关的碘通量低1至2个数量级(表 1; Lu et al., 2010及其引用的文献)。高生产力区域混合层水体内的生物泵作用, 会造成水体中碘酸盐浓度降低, 但大部分被有机质吸收的碘, 都会随着有机质在氧化水柱中的分解而被重新释放到海洋(Lu et al., 2010, 及其引用的文献)。因此, 一般认为海洋的碘库, 在显生宙期间大体稳定。
| 表 1 据海水输入/输出量估算的碘通量与水柱中有机质循环、火山排放碘通量的对比(据Lu et al., 2010, 有修改) Table 1 Comparison of iodine fluxes estimated from in/out of seawater with those recycled by organic matter in water column and those released by volcanic emission(modified from Lu et al., 2010) |
然而, 前寒武纪海洋的碘库, 很可能由于缺乏或仅含有有限的富碘藻类, 而比现代或显生宙的碘库整体偏大(Hardisty et al., 2017)。此外, 显生宙全球性的氧化还原转化和有机碳埋藏事件, 也很可能导致碘库的变化。例如, 初级生产力的上升, 将导致从混合层输出的有机碘增加, 从而降低总无机碘的浓度, 进而降低碳酸盐岩的I/(Ca+Mg)值(Zhou et al., 2015)。
如果在事件期, 碳酸盐岩的I/(Ca+Mg)值主要受控于碘库的变化(有机碳埋藏和大陆风化变化)而非氧化还原状态的变化, 那么, 不同剖面中的I/(Ca+Mg)值应该具有相似的变化趋势。但实际情况并非如此。如在白垩纪森诺曼阶— 土仑阶(Cenomanian-Turonian)的OAE2事件期, I/(Ca+Mg)值在不同剖面上有显著差异, 而且I/(Ca+Mg)值与风化指标钙、锂及碳同位素变化趋势并不一致。因此, 碳酸盐岩的I/(Ca+Mg)值, 主要受水体的氧化还原状态控制, 而与有机碳埋藏或风化相关的全球海洋碘库波动的影响, 均会被局部或区域性水体的氧化还原信号所覆盖(Zhou et al., 2015)。
由于碘离子是缺氧水体中碘的唯一存在形式(Wong and Brewer, 1977; Wong et al., 1985; Kennedy and Elderfield, 1987a, 1987b; Luther Ⅲ and Campbell, 1991; Farrenkopf et al., 1997; Rue et al., 1997; Farrenkopf and Luther Ⅲ , 2002), 但它不能进入碳酸盐矿物内(Lu et al., 2010), 因此, 碳酸盐岩成岩作用或原生碳酸盐矿物在缺氧孔隙水中重结晶, 都会导致全岩的I/(Ca+Mg)值下降(Hardisty et al., 2017)。古代碳酸盐岩样品中碘的存在, 可作为其沉积水体氧化条件的有力证据。
对新近纪样品的研究表明, 白云岩化作用会显著降低样品的I/(Ca+Mg)值(Hardisty et al., 2017)。但由于白云岩相较于其他类型碳酸盐岩具有更长期的稳定性, 因此早期成岩阶段形成的白云岩, 被认为是比灰岩更可靠、甚至首选的古海洋化学条件记录载体。事实上, 元古宙碳酸盐岩中高的I/(Ca+Mg)值, 经常发现于白云石中, 而不是方解石中。由于晚期成岩改造流体(如缺氧卤水, Derry, 2010)不可能含有高的I
因此, 成岩作用不可能导致碳酸盐岩中I/(Ca+Mg)值的增加, 同一地层剖面中同一层位样品的I/(Ca+Mg)最大值, 反映的是该层位局部海水的实际I
碳酸盐岩的I/(Ca+Mg)值, 是指示其沉积期海水氧化还原条件的重要指标。这个指标的开发和利用, 弥补了长期以来碳酸盐岩中指示氧化还原条件指标的不足, 显著拓展了古海洋氧化还原条件研究的方法。由于I
当I/(Ca+Mg)=0μ mol/mol时, 表明I
当0< I/(Ca+Mg)< 1.5μ mol/mol时, 表明I
当1.5< I/(Ca+Mg)< 2.5μ mol/mol时, 表明海水中[O2]> 10μ M, 超过了初级生产力所能产生的表层海水氧浓度最大值, 但同时也表明下伏或相邻水体中存在OMZ([O2]< 20~70μ M)。
当I/(Ca+Mg)> 2.5μ mol/mol时, 表明海水的氧化较充分, 下伏或相邻水体中不存在OMZ([O2]> 20~70μ M)。
应该说明, 这些临界值, 是依据现代海洋中缺氧盆地或OMZ内碘组分与氧含量的相关性研究以及方解石合成实验结果分析而提出的。由于地质历史时期海水物理化学条件可能与现代海洋存在显著差异(如碘库规模、海水氧化还原分层), 就有可能导致地质历史时期的这些临界值与现代海洋的状况存在一些偏差。因此, 将这些临界值应用于古代海水氧化还原条件的定量分析, 仍处于尝试阶段。对现代海洋碘氧化还原过程以及古代海洋碘组分的进一步深入研究, 将有可能使这些指标的应用更加可信。
作者声明没有竞争性利益冲突.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|